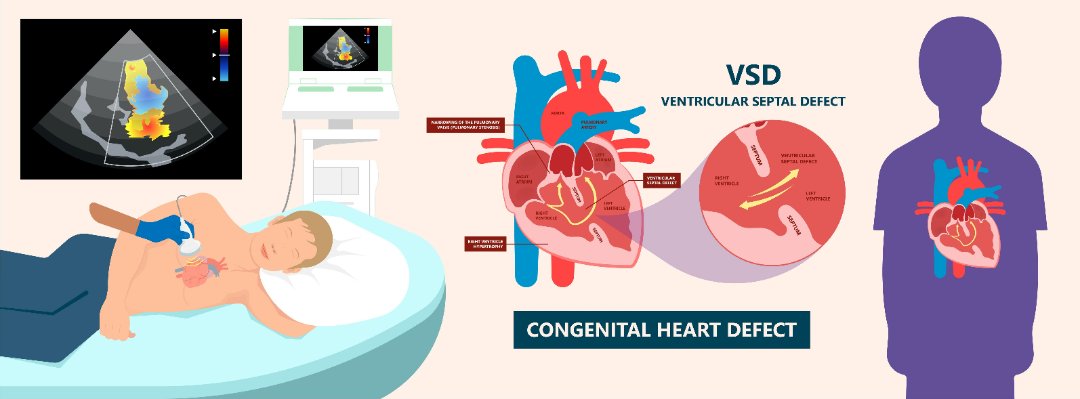
Congenital heart diseases are diseases that are present from birth and affect the normal heart functioning. These are the most frequently occurring congenital disorder, responsible for 28% of all congenital birth defects. The birth prevalence of CHD is reported to be 8-12/1000 live births. Considering a rate of 9/1000, about 1.35 million babies are born with CHD each year globally.
What are the Causes of Congenital Heart Defects?
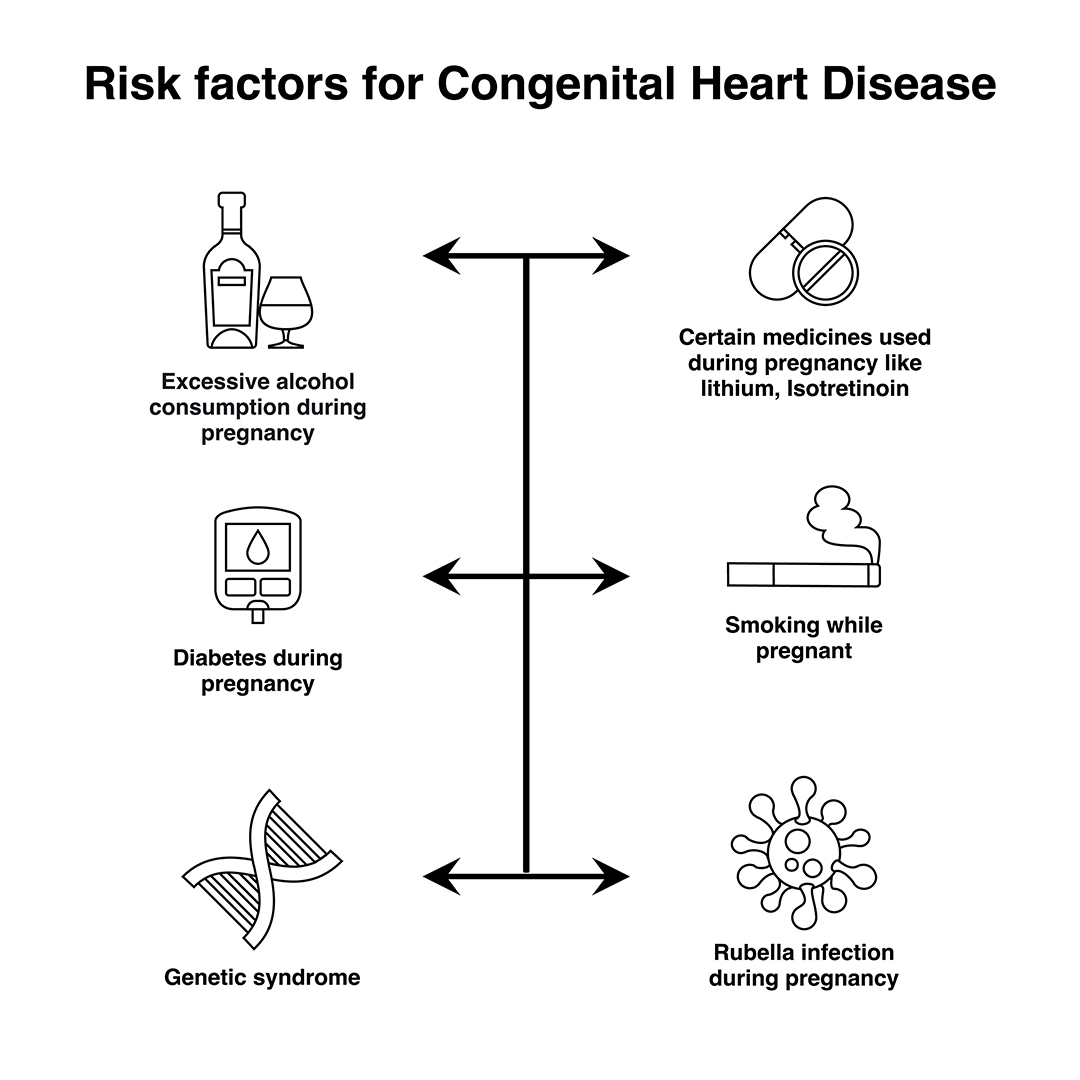
In most cases, no obvious cause of congenital heart disease is identified. However, some things are known to increase the risk of the condition, including:
- Down’s syndrome – a genetic disorder that affects a baby’s normal physical development and causes learning difficulties
- the mother having certain infections, such as rubella, during pregnancy
- the mother taking certain types of medicine during pregnancy, including statins and some acne medicines
- the mother smoking or drinking alcohol during pregnancy
- the mother having poorly controlled type 1 diabetes or type 2 diabetes
- other chromosome defects, where genes may be altered from normal and can be inherited (run in the family)
Many cases of congenital heart disease can be diagnosed before a baby is born during an ultrasound scan in pregnancy. At times a focused foetal heart scan called foetal echocardiography in specialized centers helps to diagnose the cardiac problem before birth. However, it’s not always possible to detect congenital heart defects in this way.
Signs and Symptoms of Congenital Heart Disease
Congenital heart disease can have a number of symptoms, particularly in babies and children, including:
- Rapid heartbeat
- Rapid breathing
- Swelling of the legs, tummy or around the eyes
- Extreme tiredness and fatigue
- A blue tinge to the skin or lips (cyanosis)
- Tiredness and rapid breathing when a baby is feeding
These problems are sometimes noticeable soon after birth, although mild defects may not cause any problems until later in life.
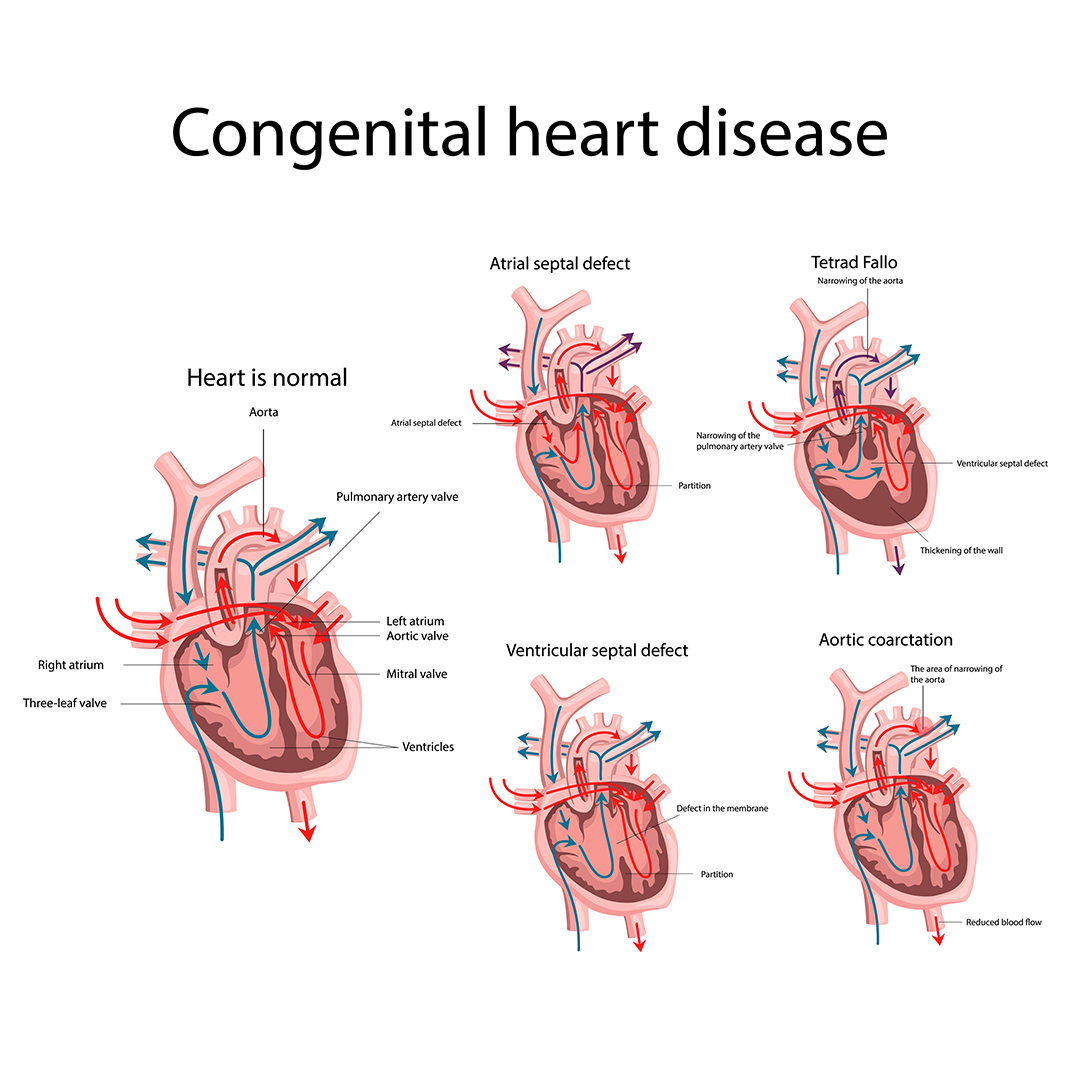
Types of Congenital Heart Disease
There are many types of congenital heart disease and they sometimes occur in combination. Some of the more common defects include:
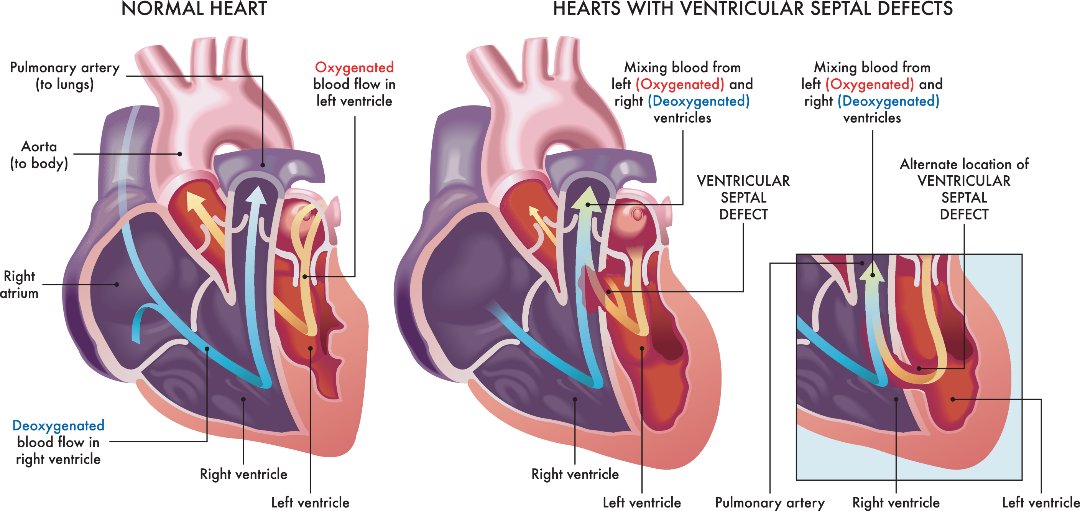
- Septal defects – where there’s a hole between 2 of the heart’s chambers (commonly referred to as a “hole in the heart”)
- Coarctation of the aorta – where the main large artery of the body, called the aorta, is narrower than normal
- Pulmonary valve stenosis – where the pulmonary valve, which controls the flow of blood out of the lower right chamber of the heart to the lungs, is narrower than normal
- Transposition of the great arteries – where the pulmonary and aortic valves and the arteries they’re connected to have swapped positions
- Underdeveloped heart – where part of the heart doesn’t develop properly making it difficult for it to pump enough blood around the body or lungs.
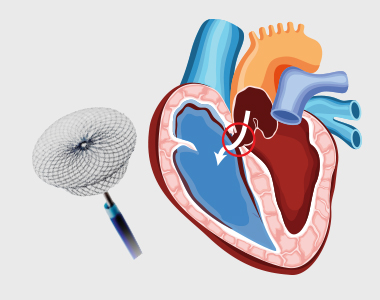
Treating Congenital Heart Disease
Treatment for congenital heart disease usually depends on the defect you or your child has.
Mild defects, such as holes in the heart, often don’t need to be treated, as they may improve on their own and may not cause any further problems.
Surgery or interventional procedures are usually required if the defect is significant and causing problems. Modern surgical techniques can often restore most or all of the heart’s normal function.
However, people with congenital heart disease often need treatment throughout their life and therefore require specialist review during childhood and adulthood. This is because people with complex heart problems can develop further problems with their heart rhythm or valves over time.
Most surgery and interventional procedures aren’t considered to be a cure. The affected person’s ability to exercise may be limited and they may need to take extra steps to protect themselves from getting infections.
It’s important that a person with heart disease and their parents or carers discuss these issues with their specialist medical team.
Book Online Consultaion
Basics of Congenital Heart Disease Blog
Subscribe the Hearty Life Blogs

DR. RAGHU | Best Cardiologist in Hyderabad
Cardiology Coronary, Vascular and
Structural Interventions
Conditions & Diseases
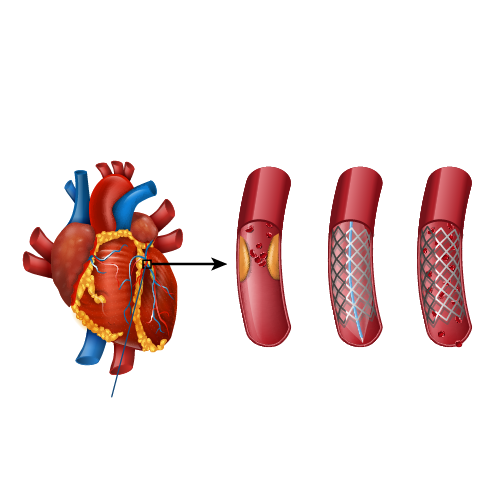
Angioplasty
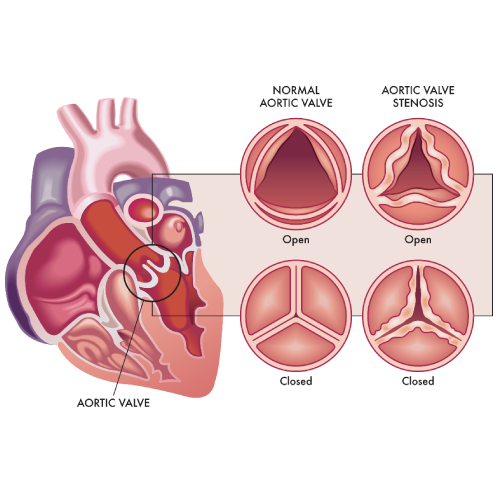
Aortic Stenosis
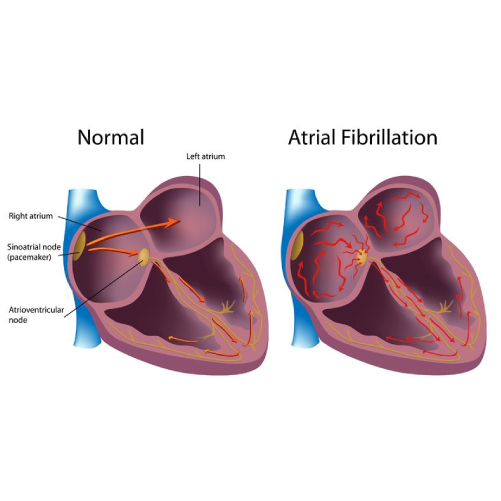
Atrial Fibrillation